Antiviral Copolymer Therapeutics
Maroon is developing a novel class of antiviral block copolymers. They adhere to hydrophobic viral surface proteins and limit a virus’ ability to infect cells, including the SARS-CoV-2 virus. Maroon’s copolymers have the potential to address several unmet needs in the area of vaccine-based antiviral agents.
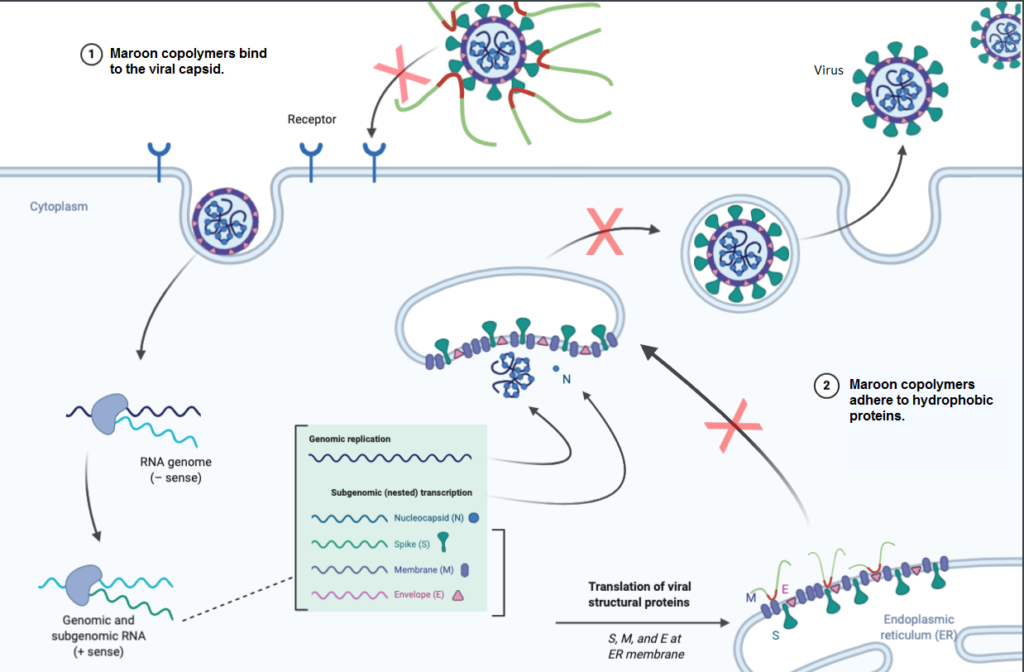

Viral infection involves binding to a host’s cell membranes, entering the cell, and inducing a stress response that drives viral replication. Maroon’s scientists postulated that certain biocompatible block copolymer surfactants would inhibit these mechanisms by:
- Hydrophobic binding to the viral capsid to limit the rate of cell entry and
- Adhering to replicated viral proteins to inhibit the unfolded protein response.
Maroon copolymers have demonstrated efficacy in treating coronaviruses, including SARS-CoV-2, in preclinical controlled testing. Comparable results are expected in other viruses with similar capsid structures. Maroon technology is expected to be less sensitive to viral mutations. This therapeutic approach has advantages in controlling future viral outbreaks.
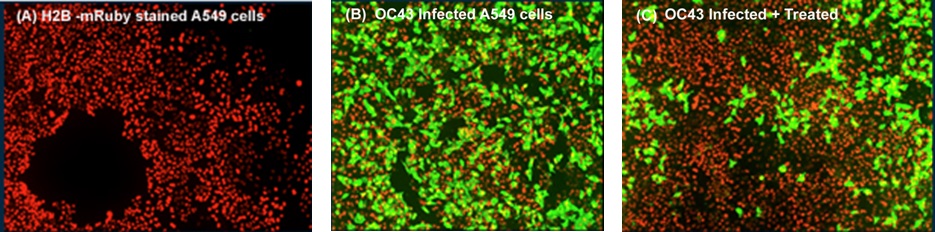

In the experiment above, human lung A549 cell lines were infected with the OC43 coronavirus strain. Infected cells stained red (left), uninfected mock control cells stained green (middle), and infected cells treated with block copolymer revealed less infection (right). Maroon antiviral copolymer exhibited efficacy in limiting viral replication and improving cell survival.
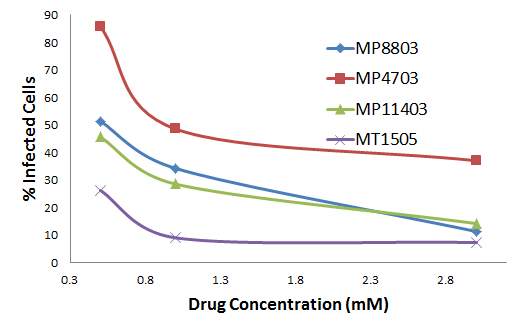
In studies of Vero E6 cells expressing SARS-CoV-2 spike proteins (right), four Maroon copolymers were found to exhibit encouraging dose responses. Results are normalized to an untreated control.

Advantages of Maroon's copolymer antiviral therapeutics:
- Mutation insensitive
- Multifunctional therapeutics for emerging viruses and future pandemics
- Antiviral therapy independent of immune function
- Potential value for immunocompromised patients
- Therapeutic option for patients with vaccine intolerance
Current Development Stage
- Maroon is conducting further preclinical testing in BSL3 COVID models.
- Intellectual property information: PCT US2021-049266.
